1. Introduction
Water pollution is one of the most important causes of environmental pollution among the various other forms of environmental degradation and it is an important climatic issue worldwide. Many scientific teams around the globe are working to reduce it by eliminating dangerous contaminants from wastewater. Eutrophication and similar challenges arise prominently from the textile, paper, leather tanning, cosmetics, pharmaceutical, and food industries, which use a large quantity of dyes and similar materials, which not only create a wastewater problem, but their refractory nature is also resistant to biodegradation [
1]. According to a report, more than 10,000 different dyes and pigment materials are used for various applications in these industries [
2]. The toxicity, carcinogenesis, and mutagenic and teratogenic properties of these water-soluble dyes make them harmful and a threat to the environment [
3]. Before discharging, conventional treatment methods, such as coagulation, flocculation, precipitation, filtration, and disinfection, turn these complex aromatic structure dyes into secondary pollutants. Therefore, there is a need to adopt an alternative method that could lead to a decrease in secondary pollutants and degrade these dyes effectively in a way that meets discharge standards. However, a single treatment method for all types of dyes is difficult to find due to their complex molecular structure.
The mineralization of the dye converts the toxic dye into non-toxic inorganic salts, water, and carbon dioxide by means of photodegradation, akin to a green chemistry approach in which visible light acts as a source of activation because strong adsorption of the dye occurs in the visual region. Moreover, the basis for the classification of the dyes as cationic, anionic, and non-ionic dyes is their molecular structure. The dyes rhodamine B (RhB) and bromophenol blue (BPB), which are commonly used in the textile industry, were chosen for this study; their molecular structures are shown in
Figure S1. The catalyst-assisted process includes the generation of free radicals. These include hydroxyl radicals (
•OH) and superoxide radicals (
•), as well as a redox reaction at the surface catalyst.
TiO
2 and ZnO nanoparticles (NPs) are established alternative heterogeneous nanophotocatalysts that are widely used for the decolorization of organic pollutants via advanced oxidation processes (AOP), and semiconductor-assisted AOP is a promising alternative method, but it has some limitations and advantages over traditional methods. Various other photocatalysts with different photodegradation mechanisms have been reported, such as UV-vis irradiation, which was used to evaluate the photodegradation of rhodamine B (RhB) on TiO
2 films. For RhB, the films showed quite different photodegradation processes. The separation of photogenerated electron holes is thought to be aided by defect sites on the surface or interface of TiO
2 films [
4]. Similarly, metal-organic frameworks (MOFs) are commonly used for promising applications. Hu et al. reported a simple and effective method to produce MOF-based melamine foams (MOFiths) with high loading stability and easy separation. By adopting this approach, various sizes of MOFs with controllable loading ratios could be fabricated. The reported MOFiths are used in the removal of organic pollutants and can be rapidly degraded using a series of cycles starting from 10 min [
5]. However, challenges in recovery and deployment conditions make MOF-based materials difficult to sustain. Furthermore, C
3N
4 has been used as a photocatalyst for degradation, as previously reported in the literature; e.g., the direct solar energy-harvesting of ammonia is a promising technique used to reduce the ammonia content in water. The energy-resolved distribution of electron traps (ERDT) around the conduction band’s bottom position was used for the first time by Chuaichama et al. to identify the structure of undoped C
3N
4 (CBB). The ERDT/CBB pattern is used to categorize the many types of elemental doping in C
3N
4 that affect photocatalytic efficiency for the reduction of Cr(VI) [
6]. Wang et al. introduced a new type of atomic single-layer graphitic-C
3N
4, which has good photocatalytic activity. In addition, the results indicated that over 80% of TAN could be removed in 6 h by means of Xe lamp irradiation. High photocatalytic activity of the solution was also exhibited by the SL g-C
3N
4 [
7]. Under visible light irradiation, an Fe-based ionic liquid-doped g-C
3N
4 (Fe-CN) photocatalyst was able to successfully degrade rhodamine B (RhB). Fe-doping has the potential to greatly speed up the separation of photogenerated carriers. It has been claimed that the synergistic impact of
• and
•OH aided in the effective degradation of RhB [
8]. Graphene-based materials are regaining popularity as innovative materials for environmental applications. The unique physicochemical features of these materials could lead to new or enhanced technologies to address the most critical environmental issues facing the world. Graphene could be employed as a component in next-generation water treatment and desalination membranes, as well as as an electrode material for contamination detection and removal [
9]. Moreover, the photocatalytic degradation of trimethoprim has been demonstrated through the hydrothermal production of Cu
2O nanostructures (TMP). The specific activity of 1.12 µmol·g
−1·min
−1 for a 0.1 mM TMP aqueous solution is comparable to prior results requiring higher energy and/or intensity UV irradiation. A hierarchical Cu
2O nanostructure demonstrated the highest photodegradation activity. The hydroxymethyl radicals photogenerated over the Cu
2O nanostructure induce the stepwise hydroxylation and oxidative cleavage of TMP to yield monocyclic fragments [
10]. A nanohybrid of CuO/Mn
3O
4/ZnO was developed by Raha et al. using a simple hydrothermal technique. When irradiated with visible light, the hybrid photocatalyst caused the 97.02% degradation of rabeprazole. The photodegradation progressed in accordance with the pseudo-first-order kinetic model, with a velocity constant of 0.07773 min
−1 [
11]. By inhibiting the recombination of photogenerated electron-hole pairs, a combination of Zn-Ti-layered double hydroxide/montmorillonite (ZTL/MT) and iron (Fe)-doped MT could improve photocatalytic activity. Fe impurities in MT form a novel mid-gap state that promotes a Z-scheme heterojunction with ZTL, resulting in reduced charge carrier recombination in ZTL. These findings show that MT can not only operate as a support material, but also as a photocatalyst for Cr(VI) reduction [
10].
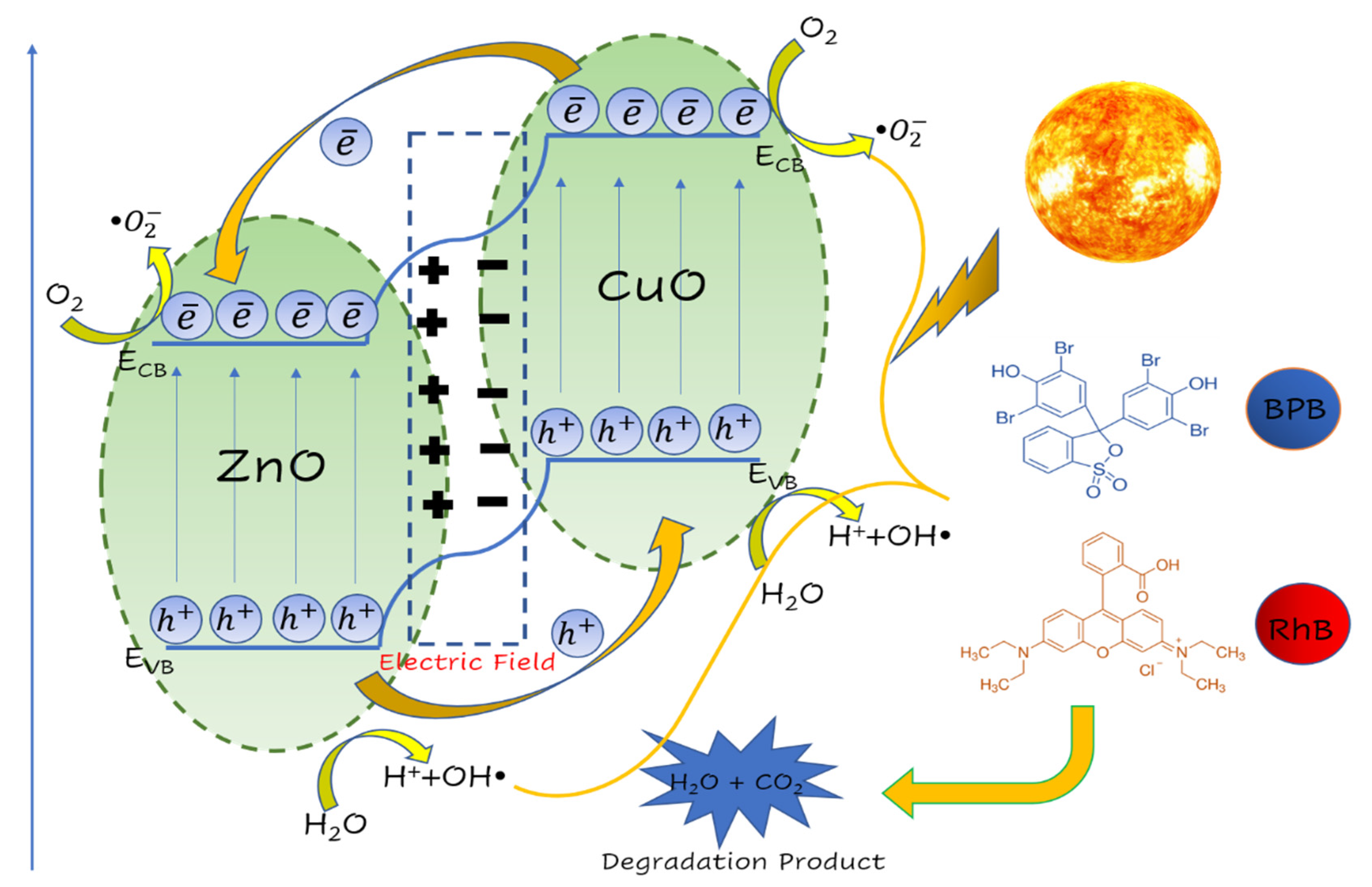
At ambient temperatures, the ZnO nanoparticle, as a semiconductor with a broad bandgap (~3.2 eV), displays a high exciton binding energy (60 meV). Furthermore, in the presence of UV photon energy, electrons become excited from the valence band (VB) to the conduction band (CB), thereby, limiting its potential application in pollutant cleanup applications. Consequently, it is important to design and tune the ZnO crystal structure and morphology and lower the electron-hole (ē/h+) pair recombination rate for viable photodegradation under visible-spectrum light by means of a suitable dopant or additive. As a result, one of the current research hotspots has been the development of highly effective and environmentally-friendly photocatalysts. A combination of two semiconductive materials is the most attractive approach for enhancing the photocatalytic responses and reducing electron-hole pair recombination. A narrow-bandgap (~1.3 eV) monoclinic CuO was suitable to enhance the photocatalytic performance of ZnO among the various narrow-bandgap metal oxide semiconductor nanomaterials studied because CuO has grown in popularity as a material for gas sensing, supercapacitors, rechargeable lithium-ion batteries, photovoltaic applications, solar hydrogen processing, and catalytic activities. In addition to being synthesized in an eco-friendly manner and readily available, the energy gaps of ZnO (3.2 eV) and CuO (1.3 eV) cover the ultraviolet and visible spectra, which proves their efficient candidacy for the catalytic degradation of dyes. Among numerous synthesis strategies, such as co-precipitation, sol-gel, hydrothermal, solid-state based synthesis, and the solution combustion method, the co-precipitation synthesis method using a simple aqueous solution approach, free from a template and surfactant, was used for the synthesis of ZnO/CuO.
The study of experimental designs based on response surface methodology (RSM) or the Box–Behnken design (BBD) is a valuable technique since it generates a mathematical model that aids in the optimization of various factors and their interactions [
12,
13]. Furthermore, it requires fewer tests, saves time, and thus minimizes errors and the amount of chemicals and manpower needed. Previously reported data showed correlations between the theoretically predicted and experimentally generated results [
14,
15]. Motivated by this fact, the prime focus of the present study was to statistically optimize the process conditions for RhB and BPB dye degradation and to evaluate the optimal conditions for dye degradation.
No study has yet been carried out, based on the authors’ best knowledge, in which ZnO/CuO was synthesized at these ratios and their photocatalytic output under visible light was optimized by utilizing the response surface methodology in correlation with central composite design. The prime objective of this work was to (a) easily synthesize CuO, ZnO, and their nanocomposite ZnO/CuO; (b) investigate their morphological and structural properties by employing scanning electron microscopy (SEM) and the X-ray diffraction technique (XRD); (c) compare, evaluate, and optimize their photocatalytic activity under the influence of visible light irradiation against RhB (cationic) and BPB (anionic) using RSM based on CCD.
3. Experimental Methods
3.1. Materials
In this experiment, ACS-grade copper (II) acetate monohydrate (Cu(CH
3COO)
2·H
2O) (≥99%, Merck, New Delhi, India), zinc acetate dihydrate (Zn(CH
3COO)
2·2H
2O) (≥99%, Merck, New Delhi, India), and sodium hydroxide pellets (≥95%, Merck, New Delhi, India) were used as received. Rhodamine B (RhB, C
28H
31ClN
2O
3) and bromophenol blue (BPB, C
19H
10Br
4O
5S) dye, procured from Thermo Fisher Scientific (New Delhi, India), were used as model organic contaminants, and their chemical structures are shown in
Figure S1. Furthermore, to prepare the solutions, double distilled water (DD) was used throughout the experiment.
3.2. Preparation of ZnO/CuO Nanocomposite
The calculated amounts of zinc acetate and copper acetate solutions were mixed with varying w/w ratios, whereas NaOH (8N, 40 mL) was added under stirring until pH = 10. The reaction was continued at 80 °C for 3 h with constant stirring. The resulting precipitate was then filtered and washed several times with a 2:1 ethanol/water solution. This was completed to remove the impurities from the sample, which was then dried overnight in a hot air oven with the temperature set to 80 °C. Finally, the sample was ground into powder and calcined at 500 °C for 3 h. ZC1, ZC2, ZC3, ZC4, and ZC5 were the names for samples of varying proportions of zinc and copper, at 90:10, 70:30, 50:50, 30:70, and 10:90, respectively. Pure ZnO and CuO were also prepared under analogous conditions to those mentioned above using separate solutions, for comparing the photocatalytic efficiency against BPB and RhB dye.
The set of reactions that express the overall process is as follows:
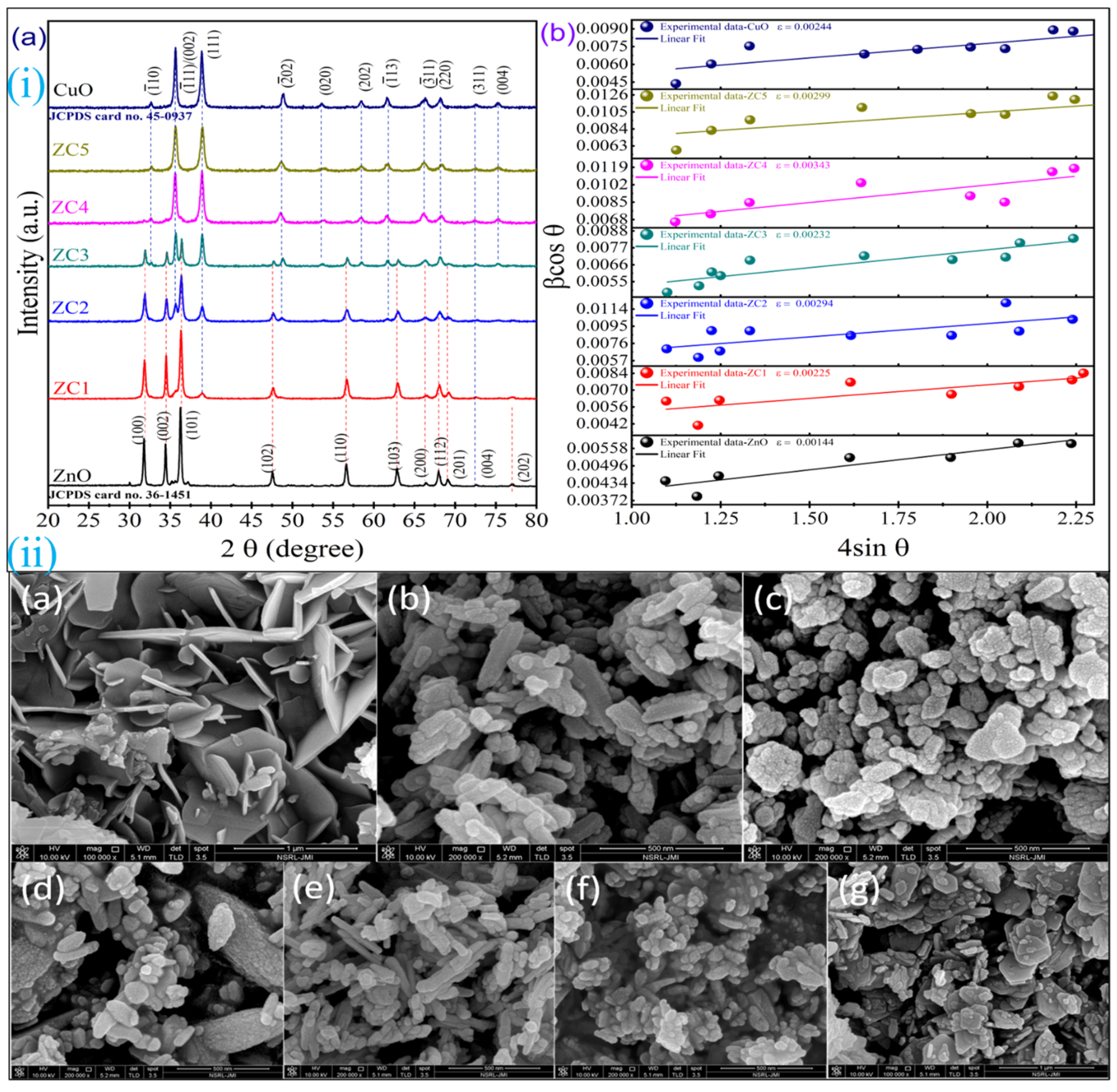
3.3. Characterizations
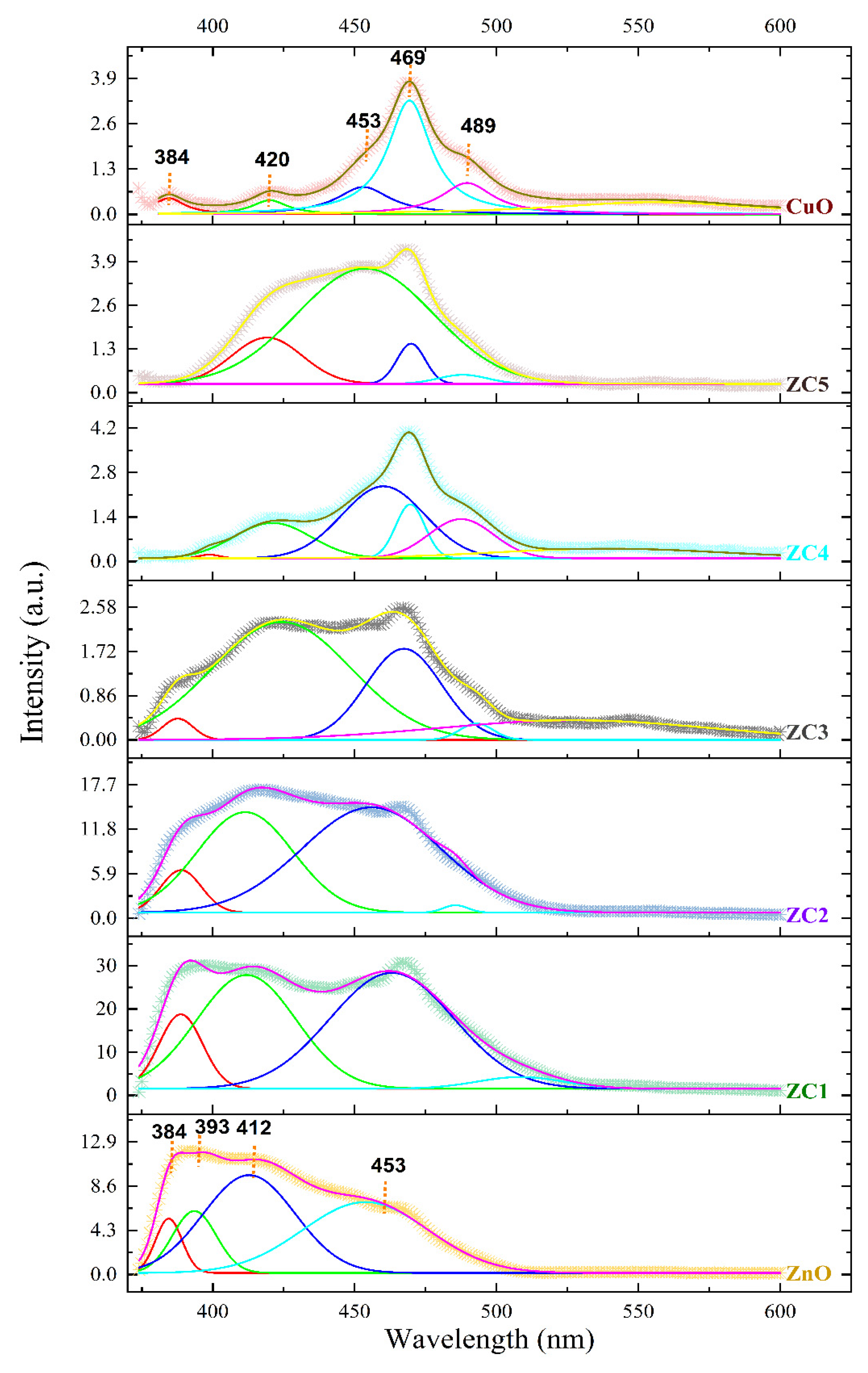
The structural properties and crystallinity of the synthesized nanomaterials were examined using an advanced powder X-ray diffractometer (XRD, Rigaku, Tokyo, Japan) under the operating conditions of 40 kV along with monochromator Cu Kα radiation (λ = 1.54 Å) and 2θ (degree) having a range from 20° to 80°. The surface morphology was analyzed using a Field emission scanning electron microscope (FE-SEM, Zeiss, Oberkochen, Germany, model number sigma 500 VP). The results for the functional group with the Fourier transform infrared (FT-IR, Perkin Elmer, Waltham, MA, USA) spectra were recorded using the sample pellets with KBr in a transmittance mode in the 400−4000 cm−1 wavenumber range. A Brunauer–Emmett-Teller (BET) surface area analyzer (Quanta Chrome Novae-2200, Boynton Beach, FL, USA), operated at 150 °C for 3 h, was used to determine the specific surface area and the pore size distribution. In relation to the optical properties, these were probed using a UV-vis spectrophotometer (double beam) from Hitachi, Tokyo, Japan, model-U3900, and a Raman spectrometer (LabRAM, HORIBA FRANCE SAS, Longjumeau, France, Model No. HR800, JY). All photocatalytic experiments were also performed using a UV-vis spectrophotometer (double beam) from Hitachi, Tokyo, Japan, model-U3900. The study of photoluminescence (PL) was carried out using a fluorescence spectrophotometer (Shimadzu, Kyoto, Japan RF-5000) at an excitation wavelength of 350 nm to determine the energy structure of nanoparticles.
3.4. Photocatalytic Experiment
Photocatalytic experiments of ZnO/CuO against RhB (cationic) and BPB (anionic) dyes were performed under visible light irradiation. A required catalytic quantity was added to a 50 mL dye solution at natural pH = 6.0 with known initial concentrations (RhB concentration of 10 mg L
−1 and BPB concentration of 10 mg L
−1). After the sonication of the suspension for 15 min, followed by the continuous stirring for the next 30 min in the dark, the adsorption-desorption equilibrium was established. Three-milliliter aliquots were extracted and centrifuged at predetermined time intervals of up to 90 min after exposing the sample to a visible light source (LED lamp Philips energy saver 40W, New Delhi, India) [
28]. To measure the extent and progress of dye degradation, the absorbance was measured at λ
max = 553 nm (RhB) and λ
max = 591 nm (BPB) wavelengths of the supernatant, using a UV-vis spectrophotometer. All the experiments were performed at room temperature (27 °C ± 2 °C). The percentage of photocatalytic degradation efficiency (D) was calculated from the following Equation (21):
where C
0 and C
t are the initial concentration of dye (mg L
−1) and the concentration of dye (mg L
−1) after time t (min), respectively [
61].
Figure S1 shows the standard calibration curve for a known concentration of BPB and RhB dye, which was used for further calculations.
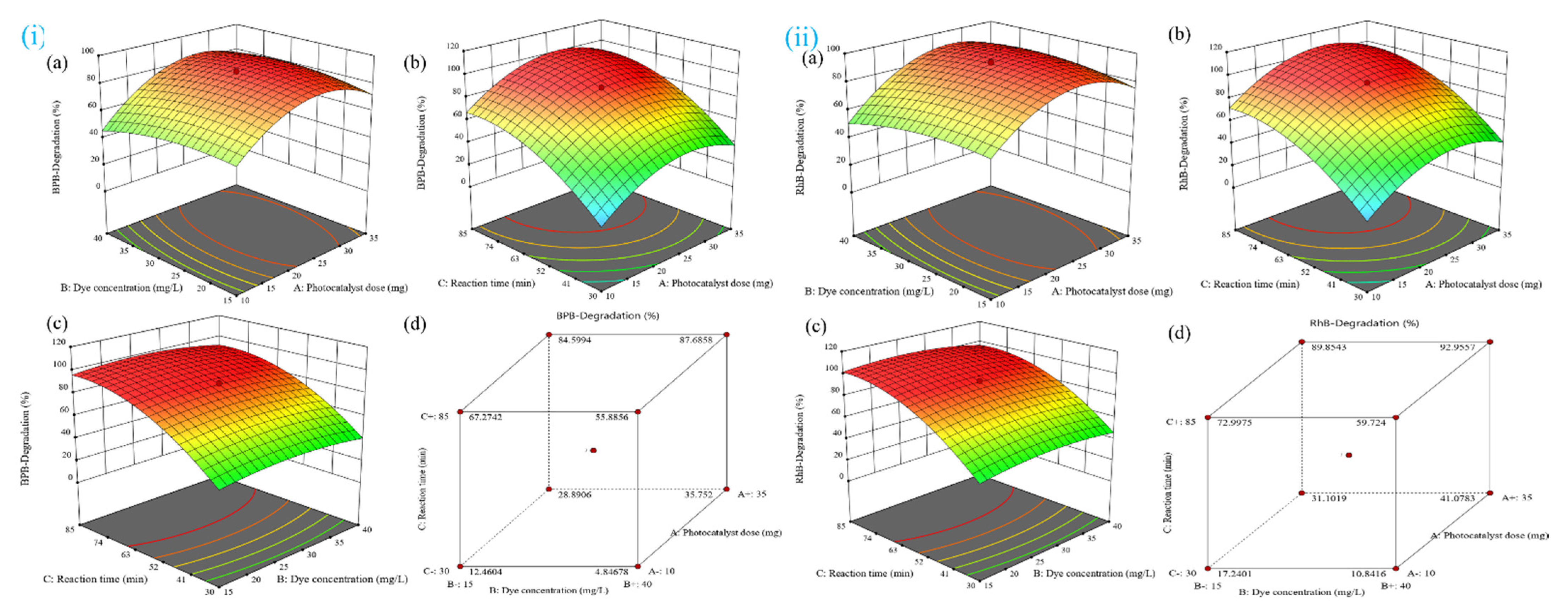
3.5. Experimental Design and Optimization Study
The optimization of the photodegradation process and the response surface modeling was performed utilizing the central composite experimental design (CCD) approach. Design-Expert 13.0.1.0 (State-Ease, Minneapolis, MN, USA) statistical software was used to analyze and interpret the experimental design. RSM was used to optimize three independent parameters, namely, the photocatalyst dose (A, mg), dye concentration (B, mg L
−1), and reaction time (C, min) for the investigation. CCD was used for all the experiments.
Table 8 lists the levels and their values, whereas
Table S1 enumerates the responses confronting the 17 combinations with six components, eight factors, and three replicates at a central point. The parameters and their values were chosen based on the results of prior experiments [
27,
62,
63]. To fit a second-order polynomial model with the experimental data, the multiple regression equation approach was adopted. The model used a second-order (quadratic) Equation (22), which is given below [
3,
64]:
where Y symbolizes the predicted response variable (Dye degradation, %); n denotes the number of analyzed factors;
is the intercept;
,
and
are the linear (main effect), quadratic and interactive model coefficients, respectively; and
and
pinpoint the level of the independent parameters being considered and analyzed.
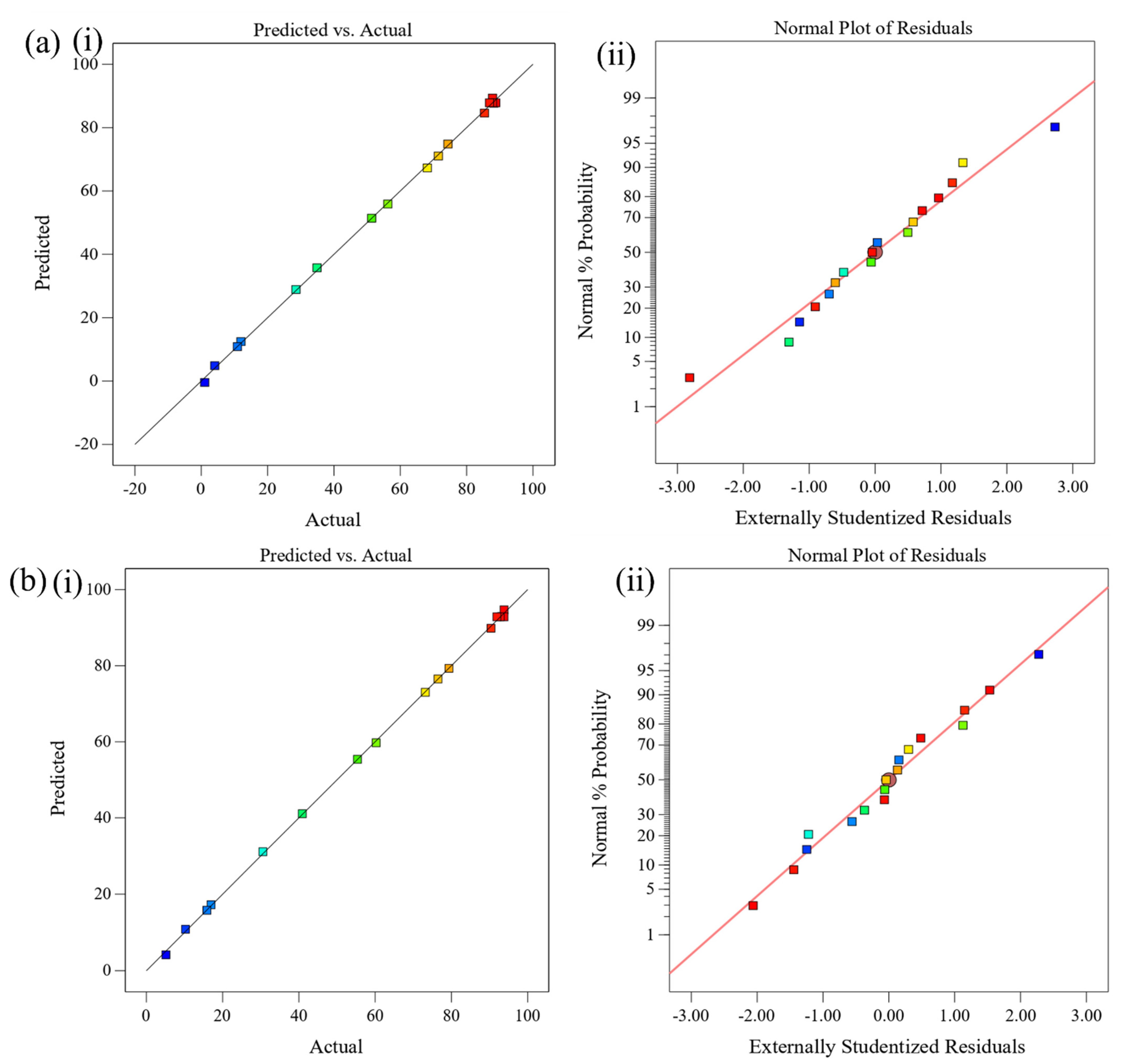
3.6. Statistical Analysis
The experimental results were statistically assessed using Design-Expert Software (version 13.0.1.0, Minneapolis, MN, USA). The goodness of fit of the regression model was evaluated using the coefficients of determination (R2), the model’s F-value (Fischer variation ratio), and the probability value (prob > F), as well as analysis of variance (ANOVA). To better understand the optimal dye degradation conditions on response variables, Design Expert Software (version 13.0.1.0, Minneapolis, MN, USA) was used to construct three-dimensional response plots.